The question is incomplete, here is the complete question:
Consider the following reaction:
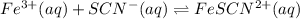 A solution is made containing an initial
A solution is made containing an initial
![[Fe^(3+)]](https://img.qammunity.org/2021/formulas/chemistry/college/9vyb68ikaq2mwz5wpztbxb86no4ye8wk3u.png) of 1.2×10⁻³ M and an initial [SCN⁻] of 8.0×10⁻⁴ M . At equilibrium, [FeSCN²⁺]= 1.8×10⁻⁴ M.
of 1.2×10⁻³ M and an initial [SCN⁻] of 8.0×10⁻⁴ M . At equilibrium, [FeSCN²⁺]= 1.8×10⁻⁴ M.
Calculate the value of the equilibrium constant (Kc).
Answer: The value of
 for above equation is 284.63
for above equation is 284.63
Step-by-step explanation:
We are given:
Initial concentration of
![[Fe^(3+)]=1.2* 10^(-3)M](https://img.qammunity.org/2021/formulas/chemistry/college/mzewfk41j2qv5de909k62pkiejf6rnuf5b.png)
Initial concentration of
![[SCN^(-)]=8.0* 10^(-4)M](https://img.qammunity.org/2021/formulas/chemistry/college/4yyhbwy0j3dc8l1sbzjo251juopja5igxk.png)
Equilibrium concentration of
![[FeSCN^(2+)]=1.8* 10^(-4)M](https://img.qammunity.org/2021/formulas/chemistry/college/tle48uyqm9n135pmkxgvyimr743yubq449.png)
The given chemical equation follows:
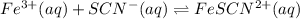
Initial:
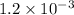
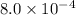
At eqllm:
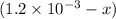
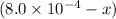 x
x
Equilibrium concentration of
![[Fe^(3+)]=(1.2* 10^(-3))-(1.8* 10^{-4)=1.02* 10^(-3)M](https://img.qammunity.org/2021/formulas/chemistry/college/sigmv4sl3d28no9u3g1z0jtt4a5ibs1gyn.png)
Equilibrium concentration of
![[SCN^(-)]=(8.0* 10^(-3))-(1.8* 10^{-4)=6.2* 10^(-4)M](https://img.qammunity.org/2021/formulas/chemistry/college/8gbb4zzde4cgloc8jh6tny7zle6bhxyaba.png)
The expression of
 for above equation follows:
for above equation follows:
![K_c=([FeSCN^(2+)])/([Fe^(3+)][SCN^-])](https://img.qammunity.org/2021/formulas/chemistry/college/n3tjtf1ifefcd1n54yq9m7s1nsfquyhq05.png)
Putting values in above equation, we get:
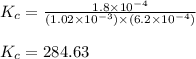
Hence, the value of
 for above equation is 284.63
for above equation is 284.63