Answer: The speed necessary for the electron to have this energy is 466462 m/s
Step-by-step explanation:
Kinetic energy is the energy posessed by an object by virtue of its motion.
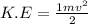
K.E= kinetic energy =
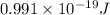
m= mass of an electron =
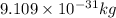
v= velocity of object = ?
Putting in the values in the equation:
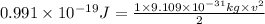
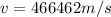
The speed necessary for the electron to have this energy is 466462 m/s