Answer:
50J
Step-by-step explanation:
For a Carnot engine, the work output (W) per cycle is given by;
W = Q (1 -
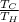 ) ----------------(i)
) ----------------(i)
Where;
Q = heat absorbed by the engine per cycle
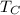 = Temperature of the colder reservoir
= Temperature of the colder reservoir
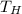 = Temperature of the hotter reservoir
= Temperature of the hotter reservoir
From the question;
Q = 100J
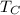 = 300K
= 300K
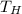 = 600K
= 600K
Substitute these values into equation (i) as follows;
W = 100 (1 -
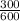 )
)
W = 100 (1 -
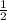 )
)
W = 100 (
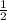 )
)
W = 50 J
Therefore, the work output per cycle is 50J