Answer:
The number of germanium atoms per cubic centimeter for this germanium-silicon alloy is 3.16 x 10²¹ atoms/cm³.
Step-by-step explanation:
Concentration of Ge (
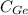 ) = 15%
) = 15%
Concentration of Si (C
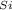 ) = 85%
) = 85%
Density of Germanium (ρ
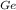 ) = 5.32 g/cm³
) = 5.32 g/cm³
Density of Silicon (ρ
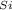 ) = 2.33 g/cm³
) = 2.33 g/cm³
Atomic mass of Ge (A
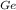 )= 72.64 g/mol
)= 72.64 g/mol
To calculate the number of Ge atoms per cubic centimeter for the alloy, we will use the formula:
No of Ge atoms/cm³=[Avogadro's Number*
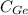 ]/([
]/([
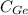 *A
*A
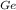 /ρ
/ρ
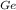 )+(C
)+(C
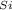 *A
*A
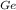 /ρ
/ρ
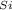 )]
)]
= (6.02x10²³ * 15%) / [(15% * 72.64/5.32)+(72.64*85%/2.33)]
= (9.03x10²²)/(2.048+26.499)
= (9.03x10²²)/(28.547)
No of Ge atoms/cm³ = 3.16 x 10²¹ atoms/cm³