The temperature of the system is 141.36K.
Step-by-step explanation:
According to the universal gas law, the pressure of a gas is directly proportional to the absolute temperature and number of moles of the gas and inversely proportional to the volume of the gas.
So in equation format,
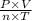 =constant.
=constant.
Or
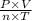 = R.
= R.
Here R is called the universal gas constant which has different values in different units.
Here the unit of volume is in litres and unit of pressure is in atmosphere.
So. Value of R comes as =0.082057
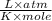 .
.
So, according to the question,
P is 5.8atm, V is 12 litres and n is 6 moles.
So,
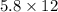 =
=
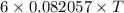 .
.
Or, T =
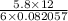 .
.
Or, T = 141.36.
So,the temperature of the system is 141.36K