Answer: The freezing and boiling points of the solution is -114.34°C and 48.95°C respectively
Step-by-step explanation:
- Calculating the freezing point:
Depression in freezing point is defined as the difference in the freezing point of pure solution and freezing point of solution.
The equation used to calculate depression in freezing point follows:
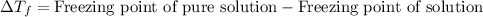
To calculate the depression in freezing point, we use the equation:
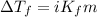
Or,
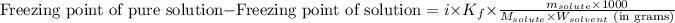
where,
Freezing point of pure solution = -110.8°C
i = Vant hoff factor = 1 (For non-electrolytes)
 = molal freezing point constant = 3.83°C/m
= molal freezing point constant = 3.83°C/m
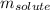 = Given mass of solute
= Given mass of solute
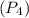 = 8.44 g
= 8.44 g
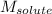 = Molar mass of solute
= Molar mass of solute
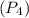 = 124 g/mol
= 124 g/mol
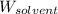 = Mass of solvent (carbon disulfide) = 60.0 g
= Mass of solvent (carbon disulfide) = 60.0 g
Putting values in above equation, we get:
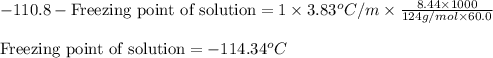
Hence, the freezing point of solution is -114.34°C
- Calculating the boiling point:
Elevation in boiling point is defined as the difference in the boiling point of solution and freezing point of pure solution.
The equation used to calculate elevation in boiling point follows:
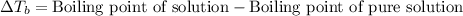
To calculate the elevation in boiling point, we use the equation:
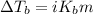
Or,
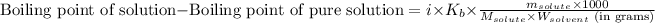
where,
Boiling point of pure solution = 46.3°C
i = Vant hoff factor = 1 (For non-electrolytes)
 = molal boiling point constant = 2.34°C/m
= molal boiling point constant = 2.34°C/m
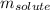 = Given mass of solute
= Given mass of solute
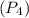 = 8.44 g
= 8.44 g
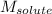 = Molar mass of solute
= Molar mass of solute
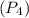 = 124 g/mol
= 124 g/mol
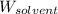 = Mass of solvent (carbon disulfide) = 60.0 g
= Mass of solvent (carbon disulfide) = 60.0 g
Putting values in above equation, we get:
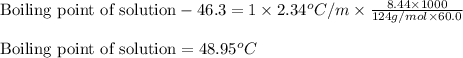
Hence, the boiling point of solution is 48.95°C