Answer:
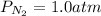
Step-by-step explanation:
Hello,
Considering the Dalton's law which states that the total pressure of a gaseous system is defined by the summation of the the partial pressures of the present gases:

For the given system:
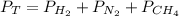
Solving for the partial pressure of nitrogen we obtain:
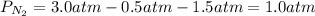
Best regards.