Step-by-step explanation:
As the given data is as follows.
m = 6.51 kg, g = 9.8
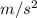 , h = 66.8 m
, h = 66.8 m
mass of water (M) = 0.68 kg, Specific heat of water = 4200
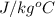
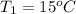
According to the given situation, the decrease in potential energy of mass will be equal to heat energy gained by water.
Therefore,
mgh =
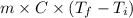
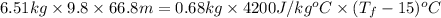
4261.7064 =
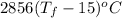
1.492 =
 - 15
- 15
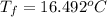
So,
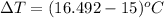
=
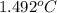
Therefore, we can conclude that rise in temperature will be
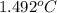 .
.