Answer : The molarity of calcium ion on the original solution is, 0.131 M
Explanation :
The balanced chemical reaction is:
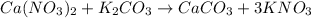
When calcium nitrate react with potassium carbonate to give calcium carbonate as a precipitate and potassium nitrate.
First we have to calculate the moles of
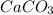
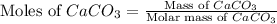
Given:
Mass of
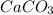 = 0.524 g
= 0.524 g
Molar mass of
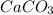 = 100 g/mol
= 100 g/mol
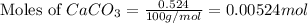
Now we have to calculate the concentration of
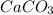
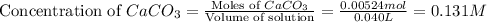
Now we have to calculate the concentration of calcium ion.
As, calcium carbonate dissociate to give calcium ion and carbonate ion.
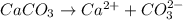
So,
Concentration of calcium ion = Concentration of
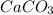 = 0.131 M
= 0.131 M
Thus, the concentration or molarity of calcium ion on the original solution is, 0.131 M