Answer:
-47.5716 kJ
Step-by-step explanation:
In an isothermal process, temperature is constant and the workdone (W) in compressing a gas from some initial pressure(P₁) and volume (V₁) to some other pressure (P₂) and volume (V₂) is given by;
W = P₁V₁ ln[
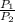 ] ---------------------(i)
] ---------------------(i)
From the question;
Nitrogen gas =
Initial and constant temperature = 420K
initial pressure P₁ = 116 kPa = 116 x 10³ Pa
initial volume V₁ = 3m³
P₂ = 455 kPa = 455 x 10³ Pa
Substitute these values into equation (i) as follows;
W = 116 x 10³ x 3 ln [
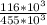 ]
]
W = 116 x 10³ x 3 ln [
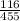 ]
]
W = 348000 ln [
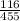 ]
]
W = 348000 x -1.367
W = -475716 J
Divide the result by 1000 to convert it to kJ
=> W = (-475716 / 1000) kJ
=> W = -47.5716 kJ
Therefore the work done in the isothermal process is -47.5716 kJ
Note: The negative value indicates that work is done on the system.