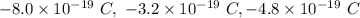Answer:
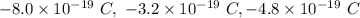
Step-by-step explanation:
Charge of an Electron
Since Robert Millikan determined the charge of a single electron is
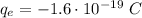
Every possible charged particle must have a charge that is an exact multiple of that elemental charge. For example, if a particle has 5 electrons in excess, thus its charge is

Let's test the possible charges listed in the question:
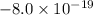 . We have just found it's a possible charge of a particle
. We have just found it's a possible charge of a particle
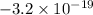 . Since 3.2 is an exact multiple of 1.6, this is also a possible charge of the oil droplets
. Since 3.2 is an exact multiple of 1.6, this is also a possible charge of the oil droplets
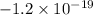 this is not a possible charge for an oil droplet since it's smaller than the charge of the electron, the smallest unit of charge
this is not a possible charge for an oil droplet since it's smaller than the charge of the electron, the smallest unit of charge
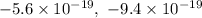 cannot be a possible charge for an oil droplet because they are not exact multiples of 1.6
cannot be a possible charge for an oil droplet because they are not exact multiples of 1.6
Finally, the charge
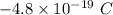 is four times the charge of the electron, so it is a possible value for the charge of an oil droplet
is four times the charge of the electron, so it is a possible value for the charge of an oil droplet
Summarizing, the following are the possible values for the charge of an oil droplet: