Answer : The value of equilibrium constant (Kc) is, 9.75
Explanation :
Now we have to calculate the value of equilibrium constant (K).
The given chemical reaction is:
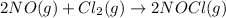
Initial conc. 0.53 0.64 0
At eqm. (0.53-2x) (0.64-x) 2x
The expression for equilibrium constant is:
![K_c=([NOCl]^2)/([NO]^2[Cl_2])](https://img.qammunity.org/2021/formulas/chemistry/high-school/82q7edd1egmo5ia6r30fdza7ls0i5waavr.png)
As we are given that:
2x = 0.36 M
x = 0.18 M
Now put all the given values in this expression, we get:
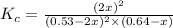
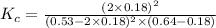

Thus, the value of equilibrium constant (Kc) is, 9.75