Answer:
Final volume: 35 L, final pressure: 0.88 atm
Step-by-step explanation:
I assume there is a typo in the question: the volume of the gas tank is constant, so the question is "what is the new pressure in the tank?"
Since the volume of the gas is constant, we can use the pressure's law, which states that:
"For an ideal gas kept at constant volume, the pressure of the gas is proportional to its absolute temperature"
Mathematically:
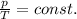
where
p is the gas pressure
T is the absolute temperature
The equation can be rewritten as
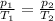
where in this problem:
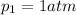 is the initial pressure
is the initial pressure
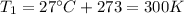 is the initial temperature
is the initial temperature
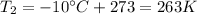 is the final temperature
is the final temperature
Therefore, the new pressure is
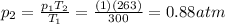
While the final volume is still 35 L.