Answer:
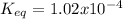
Step-by-step explanation:
Hello,
In this case, the first step is to compute the initial moles of C₅H₆O₃ as shown below:
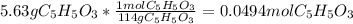
After that, by knowing that the final pressure is 1.63 atm, one computes the total moles at the equilibrium as follows:
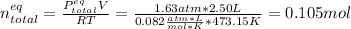
Then, by knowing the moles at the equilibrium considering the change "
 ", which yields to:
", which yields to:
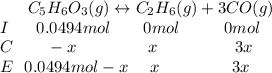
The total moles at the equilibrium turn out:
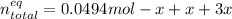
By solving for "
 ", we've got:
", we've got:
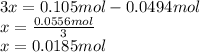
Finally, the equilibrium constant is:
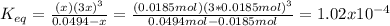
Best regards.