Answer : The mass of
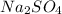 is, 4.43 grams.
is, 4.43 grams.
Explanation :
As we know that, when
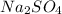 dissolve in water then it dissociates to give 2 mole of sodium ion
dissolve in water then it dissociates to give 2 mole of sodium ion
 and 1 mole of sulfate ion
and 1 mole of sulfate ion
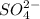
The chemical reaction will be:
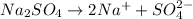
Thus, the concentration of
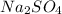 =
=
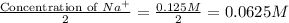
First we have to calculate the moles of
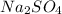
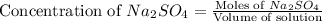
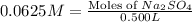
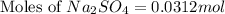
Now we have to calculate the mass of
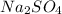
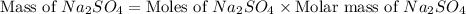
Molar mass of
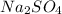 = 142 g/mol
= 142 g/mol
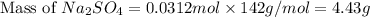
Thus, the mass of
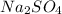 is, 4.43 grams.
is, 4.43 grams.