Answer:
51.6K
Step-by-step explanation:
Given parameters:
number of moles = 0.6moles
Volume = 1.7L
Pressure = 154.2kPa
Unknown:
temperature = ?
Solution
The ideal gas law is best used to solve this kind of problem. The law combines Boyle's law, Charles's law and Avogadro's law.
PV = nRT
wherre P = pressure in atm
V = volume in dm³ or L
n = number of moles
R = gas constant
T = temperature in kelvin
Since T is the unknown, we make it the subject of the expression;
T =
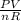
1atm = 101325Pa
x atm = 154.2 x 10³Pa
x =
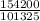 = 1.52atm
= 1.52atm
T =
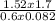 =
=
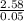 = 51.6K
= 51.6K