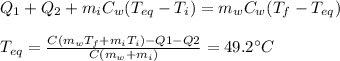Answer:
Step-by-step explanation:
As the ice is in contact with the water, heat is transferred from the water to the ice; part of the heat is used to bring the ice to its melting point (
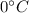 ), part is used to melt the ice, and the rest is used to increase the temperature of the ice (which is now melted) to the equilibrium temperature.
), part is used to melt the ice, and the rest is used to increase the temperature of the ice (which is now melted) to the equilibrium temperature.
At the same time, the temperature of the water decreases to the equilibrium temperature.
The heat needed by the ice to reach the melting point is:
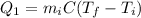
where
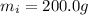 is the mass of the ice
is the mass of the ice
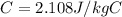 is the specific heat of ice
is the specific heat of ice
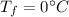 is the final temperature
is the final temperature
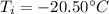 is the initial temperature
is the initial temperature
Substituting,
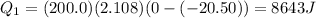
Then, the heat needed to melt the ice when it reached the melting point is
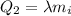
where
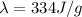 is the specific latent heat of ice
is the specific latent heat of ice
Substituting,
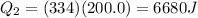
Now, the ice has completely melted, so it's now liquid water; the heat needed to reach the equilibrium temperature is
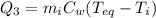
where
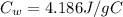 is the specific heat of water
is the specific heat of water
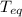 is the equilibrium temperature
is the equilibrium temperature
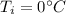 is the initial temperature of ice
is the initial temperature of ice
At the same time, the heat released by the 319.0 g of liquid water is
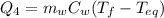
where
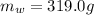 is the mass of the water
is the mass of the water
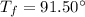 is the initial temperature of the water
is the initial temperature of the water
Since the total energy is conserved, we have:
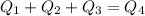
Therefore, substituting and re-arranging, we find the equilibrium temperature: