Answer:
0.028dm³
Step-by-step explanation:
Given parameters:
Molarity of Cu(NO₃)₂ = 2.36M
Volume of AgNO₃ = 250mL
Molarity of AgNO₃ = 0.519M
Unknown:
Volume of Cu(NO₃)₂ = ?
Solution:
To solve this problem, we are going to use the mole concept working from the known to the unknown specie in the balanced expression.
The known specie is the silver nitrate solution.
We should obtain our number of moles of the unknown product here and establish its volume.
Balanced equation:
Cu + 2AgNO₃ → 2Ag + Cu(NO₃)₂
Molarity =
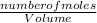
Number of moles = molarity x volume
Number of moles of AgNO₃ = ?
Convert mL to L
1000mL = 1L = 1dm³
250mL, = 0.25dm³
Number of moles of AgNO₃ = 0.519 x 0.25 = 0.13mole
From the balanced reaction equation:
2 mole of AgNO₃ = 1 mole of Cu(NO₃)₂
0.13mole of AgNO₃ =
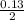 = 0.07mole of Cu(NO₃)₂
= 0.07mole of Cu(NO₃)₂
Volume of Cu(NO₃)₂ =
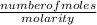 =
=
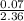 = 0.028dm³
= 0.028dm³