Answer:
1083.3kg/m ³
Step-by-step explanation:
Given parameters:
Mass of aluminium ball = 3.4kg
Apparent mass of ball = 2.1kg
Unknown:
Density of the liquid = ?
Solution:
Density is is the mass per unit volume of any give substance;
Density =

Now, we must understand that the apparent weight of the aluminium is the part of its weight supported by the fluid;
Pl =
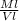
Pl = density of liquid
Ml = mass of liquid
Vl = volume of liquid
Mass of liquid = Mal - Mapparent
Mal = mass of aluminium
The volume of liquid displaced is the same as the volume of the aluminium according to Archimedes's principle;
Ml = Pl x Vl
Val = Vl
Val volume of aluminium
Vl = volume of liquid
****
Pal =
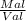
Val =
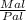
Val = volume of aluminium
Mal = mass of aluminium
Pal = density of aluminium
*****
Since the Val = Vl
Ml = Pl x
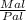
Since
Ml = Mal - Mapparent
Mal - Mapparent = Pl x
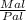
Pal = density of aluminium = 2712 kg/m ³
3.4 - 2.1 = Pl x
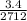
1.3 = Pl x 0.00123
Pl = 1083.3kg/m ³