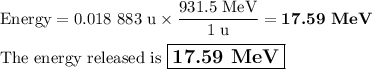Answer:
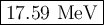
Step-by-step explanation:
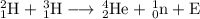
1. Calculate the mass difference
Mass of ₁²H = 2.014 102 u
Mass of ₁³H = 3.016 049
Total = 5.030 151 u
Mass of ₂⁴He = 4.002 603
Mass of ₀¹n = 1.008 665
Total = 5.011 268
Difference = 0.018 883 u
2. Convert the mass difference to energy.
931.5 MeV = 1 u