Answer:
- You could expect 3.48 grams of C₂H₄N₂
Step-by-step explanation:
You must start by stating the chemical equation for the reaction of ammonia, carbon dioxide, and methane to produce aminoaceto nitrile.
1. Word equation:
Ammonia + Carbon dioxide + Methane → Aminoacetonitrile + Water
2. Balanced chemical equation:
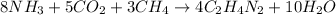
3. Convert the mass of each reactant into number of moles:
Formula:
- Number of moles = mass in grams/molar mass
2.11g NH₃
- Number of moles = 2.11g / 17.03g/mol = 0.124 mol NH₃
14.9g CO₂
- Number of moles = 14.9g/44.01g/mol = 0.339 mol CO₂
1.75g CH₄
- Number of moles = 1.75g/16.04g/mol = 0.109 mol CH₄
4. Theoretical mol ratio
From the balanced chemical equation, using the coefficientes:
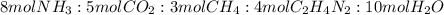
5. Limiting reagent
The available amounts of the reactants are:
Fom the theoretical mole ration, to react with 0.124 mol of NH₃ you would need:
- 0.124molNH₃ × (5molCO₂/8molNH₃) = 0.0775 mol CO₂
Since there are 0.339 moles available, this is in excess.
- 0.124molNH₃ × (3molCH₄/8molNH₃) = 0.0465mol CO₂
Since there are 0.109 moles available, this is in excess too.
Hence, the limiting reagent is NH₃.
6. Yield
Use the theoretical ratio:
- 0.124molNH₃ × (4molC₂H₄N₂ / 8molNH₃) = 0.0620 mol C₂H₄N₂
Convert to grams:
- Mass = number of moles × molar mass
- 0..0620 mol × 56.068g/mol = 3.48 g of C₂H₄N₂ ← answer