Answer:
Step-by-step explanation:
First you need to calculate the theoretical yield using the balanced chemical equation and the amount of aluminium available to react. Next, you use the percent yield to calculate the true yield.
1. Balanced chemical equation:
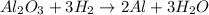
2. Mole ratios
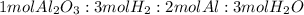
3. Number of moles of aluminium oxide
- Molar mass = 101.960 g/mol
- Mass = 700.0 kg = 7.0000 × 10⁵ g
- Number of moles = mass in grams / molar mass
- Number of moles = 7.0000 × 10⁵ g / 101.960 g/mol = 6,865.4mol
4. Number of moles of aluminium
The mole ratio is
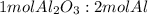 ; thus 6,865.4 mol of aluminium oxide produce 2 × 6,865.4 mol of aluminum = 13,730.8 mol of aluminium.
; thus 6,865.4 mol of aluminium oxide produce 2 × 6,865.4 mol of aluminum = 13,730.8 mol of aluminium.
5. Calculate the mass of 13,730.8 mol of aluminium
- Atomic mass of aluminium: 26.981g/mol
- Mass = atomic mass × number of moles
- Mass = 26.981g/mol × 13,730.8mol = 370,470.7g
6. Calculate the actual yield
- Actual yield = theoretical yiel × percent yield
- Actual yield = 85.0% × 370,470.7 g = 314,900.1 g
7. Convert to kg and three significant figures
- 314,900.1 g × 1kg/1,000g = 314.9kg = 315kg