The given question is incomplete. The complete question is as follows.
If gas in a cylinder is maintained at a constant temperature T, the pressure P is related to the volume V by a formula of the form
P =
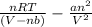 , in which a, b, n, and R are constants. Find
, in which a, b, n, and R are constants. Find
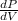 .
.
Step-by-step explanation:
We will use the quotient rule for each of the two terms on the right side as follows.
P =
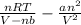
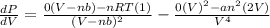
=
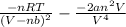
=
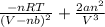
=
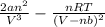
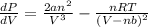
Thus, we can conclude that the value of
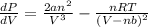 .
.