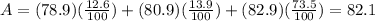Answer:
82.1
Step-by-step explanation:
Isotopes of the same element are atoms of the same element (so, having same number of protons) having a different number of neutrons (and so, a different atomic mass).
In this problem, this element has 3 different isotopes, with atomic mass (in atomic mass units, a.m.u.) and relative abundance listed below:
1) 78.9 12.6%
2) 80.9 13.9%
3) 82.9 73.5%
In order to calculate the relative atomic mass unit of this element, we have to calculate the weighed average of the relative atomic mass of each isotope.
This means that we can calculate it as the sum of each atomic mass unit multiplied by the relative frequency:
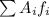
where
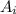 is the atomic mass of each isotope and
is the atomic mass of each isotope and
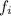 the relative frequency. Therefore, we find:
the relative frequency. Therefore, we find: