Answer: Inherent width in the emission line: 9.20 × 10⁻¹⁵ m or 9.20 fm
length of the photon emitted: 6.0 m
Explanation:
The emitted wavelength is 589 nm and the transition time is ∆t = 20 ns.
Recall the Heisenberg's uncertainty principle:-
∆t∆E ≈ h ( Planck's Constant)
The transition time ∆t corresponds to the energy that is ∆E
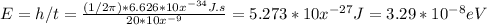 .
.
The corresponding uncertainty in the emitted frequency ∆v is:
∆v= ∆E/h = (5.273*10^-27 J)/(6.626*10^ J.s)= 7.958 × 10^6 s^-1
To find the corresponding spread in wavelength and hence the line width ∆λ, we can differentiate
λ = c/v
dλ/dv = -c/v² = -λ²/c
Therefore,
∆λ = (λ²/c)*(∆v) = {(589*10⁻⁹ m)²/(3.0*10⁸ m/s)} * (7.958*10⁶ s⁻¹)
= 9.20 × 10⁻¹⁵ m or 9.20 fm
The length of the photon (l) is
l = (light velocity) × (emission duration)
= (3.0 × 10⁸ m/s)(20 × 10⁻⁹ s) = 6.0 m