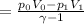Answer:
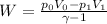
Step-by-step explanation:
An adiabatic process refers to one where there is no exchange of heat.
The equation of state of an adiabatic process is given by,
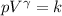
where,
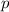 = pressure
= pressure
 = volume
= volume
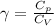
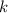 = constant
= constant
Therefore, work done by the gas during expansion is,
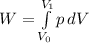
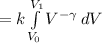
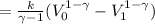
(using
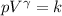 )
)