Answer:
The volume of tank is 0.25191
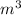 and the final pressure is 38.476 bar.
and the final pressure is 38.476 bar.
Step-by-step explanation:
mass=m=5 kg
Pressure 1=P_1=20 bar
quality=x=50% =0.5
From the steam tables for Pressure of 20 bar and the quality as 0.5
v_f=0.001177 m^3/kg
v_g=0.099587 m^3/kg
Now the specific volume is given as
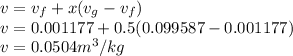
So the Total volume is given as
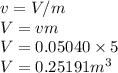
As the tank is rigid so its volume will remain same and the volume is 0.25191 m^3.
For the final pressure as the tank is rigid
v_1=v_2=0.0504 m^3/kg and saturated vapor table gives
P_sat=38.476 bar.
So the volume of tank is 0.25191
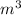 and the final pressure is 38.476 bar.
and the final pressure is 38.476 bar.