Answer:
W=-260.66 kJ (negative answer means, that the work was done on the gas)
Step-by-step explanation:
1) Convert temperature from C to K- T=24+273=297K- all temperature in the gas problems should be used in Kelvins;
2) We need to analyse type of the process- it is given, that the temperature is constant, so it is an Isothermal process, which means, that the equation of the process is: pV=const (constant);
3) Work, done on the system, should be calculated using the following equation:
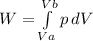
4) To calculate initical and final volumes (Va and Vb), we can use the following equation: pV=mRT, so V=mRT/p. Note, that the pressure is changing, thus we can calculate volumes for the both cases- initial and final, using initial (120kPa) and final (600kPa) pressures, in addition, we can find equation for the pressure, as function of the volume, which we need to use for the integration in step 3: p=mRT/V;
5) Now we can calculate the integral, given in the step 3:
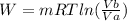 . As we have pressure as a known values, we can re-write the equation, using pressures:
. As we have pressure as a known values, we can re-write the equation, using pressures:
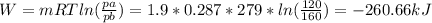
Note, that natural logarithm (ln) yields negative answer, which supports the question, that the work was done on the gas, not by the gas.