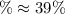Answer:
Step-by-step explanation:
The equilibrium equation is:
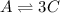
The initial concentration of A can be calculated from the ideal gas equation:

Determine the conversion of substance A to substance C using an ICE table and the Kc constant:
A ⇄ 3C
I 0.304 0
C - x + 3x
E 0.304 - x 3x
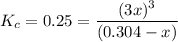
Solve for x:
You need to use a graphing calculator:
Then:
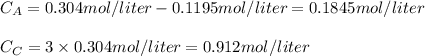
The equilbrium conversion is:
![\% = [0.304mol/liter-0.1845mol/liter]/(0.304mol/liter)* 100](https://img.qammunity.org/2021/formulas/physics/college/8x9eokuiwj2h7oeiri8dxljzi66dwhppyn.png)