The tree died about 3357 years ago.
Step-by-step explanation:
The half life of carbon - 14 is given by
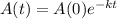
where
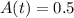
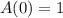
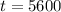
Substituting these values in the above equation, we get,
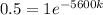
Taking In on both sides of the equation, we get,
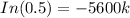
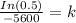
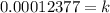
Since, only 66% of Carbon - 14 remains after the time T.
Thus, we have,
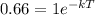
Taking In on both sides of the equation, we get,
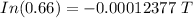
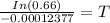
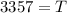
Thus, the tree died about 3357 years ago.