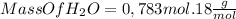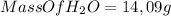Answer:
- The limiting reagent is N2O4
- 14,09g
Step-by-step explanation:
- First, we adjust the reaction.
 +
+
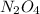 ⇄
⇄
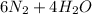
- Second, we assume that the participating moles are equal to the stoichiometric ratios because we do not know the amounts of the reagents.
We can determinate what is the limiting reagent comparing of product amounts which can be formed from each reactant.
Using
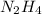 to form
to form

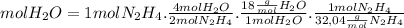
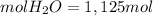
Using
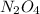 to form
to form
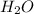
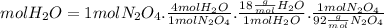
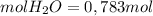
The limiting reagent is N2O4, because can produce only 0, 783 mol of H2O.
This is the minimum measure can be formed of each product.
∴