Answer: 136 g of
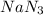 must be reacted to inflate an air bag to 70.6 L at STP.
must be reacted to inflate an air bag to 70.6 L at STP.
Step-by-step explanation:
Using ideal gas equation:

P= pressure of nitrogen gas = 1 atm (at STP)
V =volume of nitrogen gas = 70.6 L
n = number of moles of nitrogen gas = 1 atm (at STP)
R = gas constant = 0.0821 Latm/Kmol
T = temperature of nitrogen gas = 273 K (at STP)
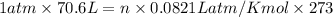
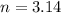
For the balanced chemical reaction:
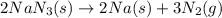
3 moles of nitrogen are produced by = 2 moles of
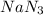
3.14 moles of nitrogen are produced by =
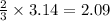 moles of
moles of
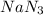
Mass of
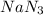 = Moles × Molar mass = 2.09 mole × 65 g/mol = 136 g
= Moles × Molar mass = 2.09 mole × 65 g/mol = 136 g