Answer: The vapor pressure of benzene and toluene at 25°C is 27.056 torr and 20.777 Torr respectively and the total vapor pressure of mixture is 47.833 Torr
Step-by-step explanation:
We are given:
Moles of benzene = 1.00 moles
Moles of toluene = 0.400 moles
Mole fraction of a substance is given by:
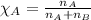
To calculate the vapor pressure of substance in a mixture, we use the equation given by Raoult's law, which is:
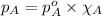 ........(1)
........(1)
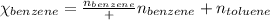
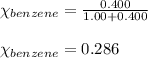
Using equation 1, we get:
Vapor pressure of pure benzene = 94.6 Torr
Putting values in equation 1, we get:
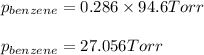
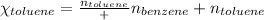
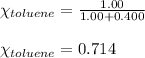
Using equation 1, we get:
Vapor pressure of pure toluene = 29.1 Torr
Putting values in equation 1, we get:
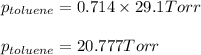
- Dalton's law of partial pressure states that the total pressure of the system is equal to the sum of partial pressure of each component present in it.
To calculate the total vapor pressure of the mixture, we use the law given by Dalton, which is:
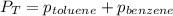
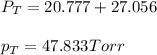
Hence, the vapor pressure of benzene and toluene at 25°C is 27.056 torr and 20.777 Torr respectively and the total vapor pressure of mixture is 47.833 Torr