Answer:
the species that could be the unknown gas is SF₆
Step-by-step explanation:
Graham's Law of Effusion states that the rate of effusion is inversely proportional to the square root of the molecular mass
hence
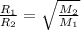
But
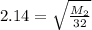 where the molar mass of oxygen O₂ = 32 we hava
where the molar mass of oxygen O₂ = 32 we hava
2.14² = M₂/32 or M₂ = 146.5472
However comparing the likely candidates it is observed that the best canditate is SF₆ as we have Sulphur molar mass = 32 and fluoriine molar mass = 18.99 then
32 + 6×18.99 = 145.95 ≅ 146