Answer:
Here's what I get
Step-by-step explanation:
(a) Lithium
i) Energy of transition line
The strong line of Li appears at 670.8 nm.
The formula for the energy of a photon is
E = hc/λ
If λ = 670.8 nm, then
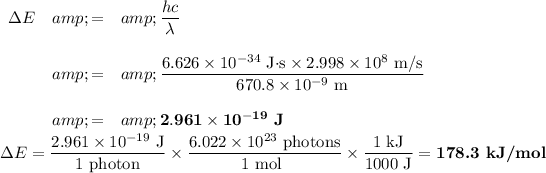
ii) Energy of Li(2p) orbital

(b) Sodium
i) Energies of transition lines
The two emission lines of Na are at 589.0 nm and 589.6 nm.
As before, we can calculate the energy of the first line as
ΔE₁ = 3.373 × 10⁻¹⁹ J/photon = 203.1 kJ/mol.
The energy of the second line is 3.369 × 10⁻¹⁹ J/photon = 202.9 kJ/mol.
ii) Energies of the sodium orbitals
The premise in your question is wrong. The two lines do not arise from 3d ⟶ 3p and 3p ⟶ 3s transitions.
Instead, the 3p orbitals are split into two energy levels by spin-orbit coupling. Both lines come from 3p ⟶ 3s transitions. These levels are 203.1 kJ/mol and 202.9 kJ/mol above the 3s level. I have diagrammed the situation in the figure below.