Step-by-step explanation:
It is known that energy balance relation is as follows.
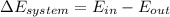
Also,
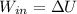
so,
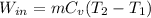
According to the ideal gas equation,
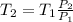
Putting the values into the above equation as follows.
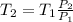
=
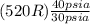
= 693.3 R
Now, we will convert the temperature into degree Fahrenheit as follows.
693.3 - 458.67
=
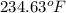
From table A-
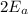
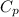 = 0.240 Btu/lbm R and
= 0.240 Btu/lbm R and
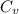 = 0.171 Btu/lbm
= 0.171 Btu/lbm
Now, we will substitute the energy balance as follows.
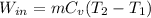
=
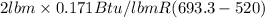
= 59.3 Btu
Thus, we can conclude that final temperature of air is 59.3 Btu.