Answer:
Part a: The total amount of energy transfer by the work done is 54.81 kJ.
Part b: The total amount of energy transfer by the heat is 54.81 kJ
Step-by-step explanation:
Mass of Carbon Dioxide is given as m1=3 kg
Pressure is given as P1=3 bar =300 kPA
Volume is given as V1=0.5 m^3
Pressure in tank 2 is given as P2=2 bar=200 kPa
T=290 K
Now the Molecular weight of
 is given as
is given as
M=44 kg/kmol
the gas constant is given as
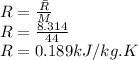
Volume of the tank is given as
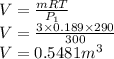
Final mass is given as
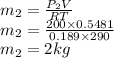
Mass of the CO2 moved to the cylinder
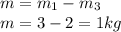
The initial mass in the cylinder is given as
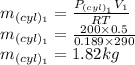
The mass after the process is
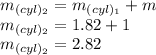
Now the volume 2 of the cylinder is given as
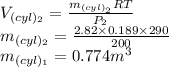
Part a:
So the Work done is given as
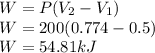
The total amount of energy transfer by the work done is 54.81 kJ.
Part b:
The total energy transfer by heat is given as
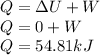
As the temperature is constant thus change in internal energy is 0.
The total amount of energy transfer by the heat is 54.81 kJ