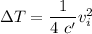Answer:
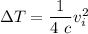
Step-by-step explanation:
Given that
mass of the bullet = m
Speed of the bullet = vi
Therefore the kinetic energy of the bullet KE will be
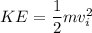
Given that 50 % of the kinetic energy is converted into thermal energy.
KE'= 0.5 KE
The thermal energy Q is given as
Q= m c ΔT
Q=Heat
m=mass
c=specific heat capacity
ΔT=Temperature difference
Therefore we can say that
Q= KE'
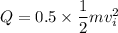
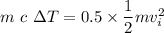
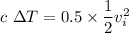
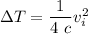
Therefore the increase in temperature is given as