Answer: The atom percent of silver, gold and copper in the alloy is 43.08 %, 44.93 % and 11.99 % respectively
Step-by-step explanation:
To convert the given masses into grams, we use the conversion factor:
1 lb = 453.6 grams
To calculate the number of moles, we use the equation:
 ......(1)
......(1)
To calculate the atom percent of substance in sample, we use the equation:
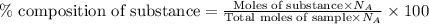 ......(2)
......(2)
where,
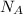 = Avogadro's number
= Avogadro's number
Mass of silver = 44.5 lb = 20185.2 grams
We know that:
Molar mass of silver = 107.87 g/mol
Putting values in equation 1, we get:
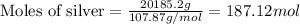
Mass of gold = 84.7 lb = 38419.9 grams
We know that:
Molar mass of gold = 196.97 g/mol
Putting values in equation 1, we get:
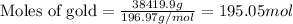
Mass of copper = 7.3 lb = 3311.3 grams
We know that:
Molar mass of copper = 63.55 g/mol
Putting values in equation 1, we get:
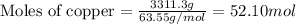
Total moles of the sample =
Moles of silver = 187.12 moles
Total moles = [187.12 + 195.05 + 52.10] = 434.27 moles
Putting values in equation 2, we get:
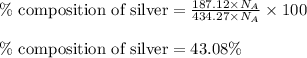
Moles of gold = 195.05 moles
Total moles = [187.12 + 195.05 + 52.10] = 434.27 moles
Putting values in equation 2, we get:
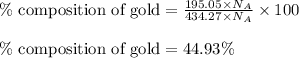
Moles of copper = 52.10 moles
Total moles = [187.12 + 195.05 + 52.10] = 434.27 moles
Putting values in equation 2, we get:
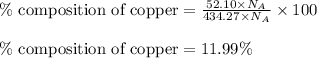
Hence, the atom percent of silver, gold and copper in the alloy is 43.08 %, 44.93 % and 11.99 % respectively