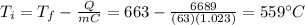Answer:
Step-by-step explanation:
When heat is supplied to a substance, the temperature of the substance increases according to:
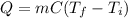
where
Q is the amount of heat supplied
m is the mass of the substance
C is the specific heat capacity of the substance
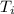 is the initial temperature
is the initial temperature
 is the final temperature
is the final temperature
For the sample of magnesium in this problem, we have:
m = 63 g is the mass
Q = 6689 J is the hear supplied
C = 1.023 J/gC is the specific heat capacity
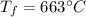 is the final temperature
is the final temperature
Solving the formula for
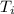 , we find the final temperature:
, we find the final temperature: