Step-by-step explanation:
(a) It is known that charge on a proton is equal to
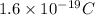 . And, net charge is given as
. And, net charge is given as
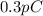 which is also equal to
which is also equal to
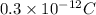 .
.
Therefore, we will calculate the number of electrons as follows.
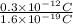
=
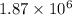
Hence, there are
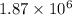 fewer electrons are there than protons.
fewer electrons are there than protons.
(b) Now, we will calculate the fraction of protons that would have no electrons as follows.
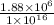
=
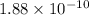
Therefore, fraction of the protons that would have no electrons is
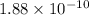 .
.