Answer: The wavelength of the photon is 486.2 nm and it lies in the visible region
Step-by-step explanation:
To calculate the wavelength of light, we use Rydberg's Equation:
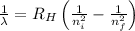
Where,
 = Wavelength of radiation
= Wavelength of radiation
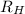 = Rydberg's Constant =
= Rydberg's Constant =
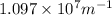
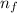 = Higher energy level = 4
= Higher energy level = 4
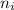 = Lower energy level = 2
= Lower energy level = 2
Putting the values in above equation, we get:
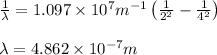
Converting this into nanometers, we use the conversion factor:
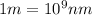
So,
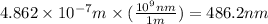
As, the range of wavelength of visible light is 400 nm - 700 nm. So, the wavelength of the given photon lies in the visible region
Hence, the wavelength of the photon is 486.2 nm and it lies in the visible region