Answer:
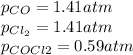
Step-by-step explanation:
Hello,
In this case, since the undergoing chemical reaction is:
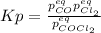
Thus, if we introduce the changing pressure,
 , the law of mass action becomes:
, the law of mass action becomes:

Now, by taking the given value of Kp we solve for x as follows:
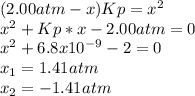
therefore, the result is 1.41 atm so the equilibrium pressures turn out:
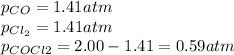
Best regards.