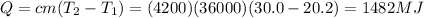Answer:
It is required 1482 Megajoules of energy.
Step-by-step explanation:
The energy Q required to raise the temperature T1 to a temperature T2 of mass m of water is:
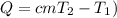 (1)
(1)
with c the specific heat of water that is
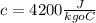 .
.
We don't have explicit the mass of water containded in the swimming pool, but we can use the relation:
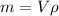
with ρ the density of water that is
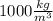 , V the volume of the swimming pool and m the mass, so m is:
, V the volume of the swimming pool and m the mass, so m is:
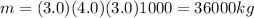
Using c and m on (1):