Answer:
D) 6
Step-by-step explanation:
since, n = molar mass / empirical formula mass
Empirical formula mass = Total mass of atoms present in empirical formula
CHCl = 12+1+35.5
= 48.5
Given, Molar mass = 290.8 g.
So, n = 290.8/48.5
= 5.995 , that is approx 6.
So, Molecular formula = n × Empirical formula
= 6 × CHCl
=
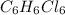
So, Number of C = 6