Answer:
The answer is "is mostly empty space".
Step-by-step explanation:
In the given question option numbers is defined, so the explanation to this question as follows:
The volume of an element holds one mole at high temperatures. The nuclear mass is usually indicated by mole: cc/mol in the
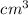 . It is also known as a quantity, in which the same molecule was the measured values based on atomic mass as well as the thickness of both, and certain alternatives were wrong which can be explained as follows:
. It is also known as a quantity, in which the same molecule was the measured values based on atomic mass as well as the thickness of both, and certain alternatives were wrong which can be explained as follows:
- atom doesn't fill the protons, neutrons, and electrons.
- It can't revolve with outside atoms and electrons.