Answer: The freezing point of solution is 5.35°C
Step-by-step explanation:
The equation used to calculate depression in freezing point follows:
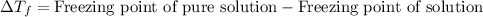
To calculate the depression in freezing point, we use the equation:
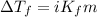
Or,
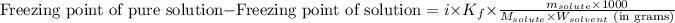
where,
Freezing point of pure solution = 5.5°C
i = Vant hoff factor = 1 (For non-electrolytes)
 = molal freezing point elevation constant = 4.90°C/m
= molal freezing point elevation constant = 4.90°C/m
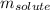 = Given mass of solute (naphthalene) = 2.60 g
= Given mass of solute (naphthalene) = 2.60 g
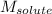 = Molar mass of solute (naphthalene) = 128.2 g/mol
= Molar mass of solute (naphthalene) = 128.2 g/mol
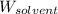 = Mass of solvent (benzene) = 675 g
= Mass of solvent (benzene) = 675 g
Putting values in above equation, we get:
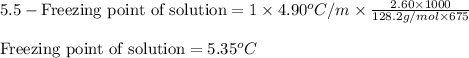
Hence, the freezing point of solution is 5.35°C