Answer:
3.01 atm
Step-by-step explanation:
According to Gay-Lussac's law, the pressure (P) of a given mass of gas when volume is left constant is directly proportional to the kelvin temperature (T) of the gas. i.e
P ∝ T
P = kT
Where;
k = proportionality constant
=>
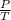 = k
= k
=>
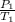 =
=
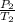 -------------------(i)
-------------------(i)
Where;
 and
and
 are the initial and final pressures of the gas
are the initial and final pressures of the gas
 and
and
 are the initial and final temperatures of the gas
are the initial and final temperatures of the gas
From the question;
 = 2.48 atm
= 2.48 atm
 = ?
= ?
 = 21°C = (21 + 273)K = 294K
= 21°C = (21 + 273)K = 294K
 = 84°C = (84 + 273)K = 357K
= 84°C = (84 + 273)K = 357K
Substitute these values into equation (i) as follows;
=>
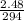 =
=
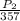
Solve for

 =
=
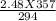
 = 3.01 atm
= 3.01 atm
Therefore the pressure will be 3.01 atm