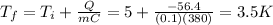Answer:
3.5 K
Step-by-step explanation:
When a sample of a substance releases heat energy, its temperature decreases according to the equation:
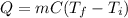
where
Q is the heat energy released
m is the mass of the substance
C is the specific heat capacity
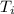 is the initial temperature
is the initial temperature
 is the final temperature
is the final temperature
For the block of brass in this problem, we have:
m = 0.1 kg is the mass
Q = -56.4 J is the heat energy released (negative since it is released)
C = 380 J/kg K is the specific heat capacity of brass
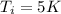 is the initial temperature
is the initial temperature
Solving for
 , we find the final temperature:
, we find the final temperature: