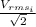Answer:
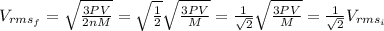
So then the final answer on this case would be:
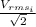
Step-by-step explanation:
From the kinetic theory model of gases we know that the velocity rms (speed of gas molecules) is given by:
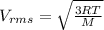 (1)
(1)
Where V represent the velocity
R the constant for ideal gases
T the temperature
M the molecular weight of the gas
We also know from the ideal gas law that
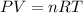
If we solve for T we got:
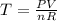
For the initial state we can replace T into the equation (1) and we got:
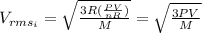
For the final state we know that :
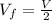 And the pressure not change , so then the final velocity would be:
And the pressure not change , so then the final velocity would be:
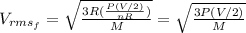
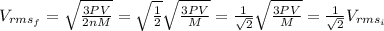
So then the final answer on this case would be: